
क्या हार में क्या जीत में ! किंचित नहीं भयभीत मैं
संधर्ष पथ पर जो मिले यह भी सही वह भी सही।
वरदान नहीं मानूंगा, हार नहीं मानूंगा |
Atomic model
Atomic models have evolved over time as scientific understanding has progressed
CHEMISTRY
10/25/20222 min read
Atomic model
To know the position of different fundamental particle in an atom it is very necessary to study the atomic model.
Important atomic models:
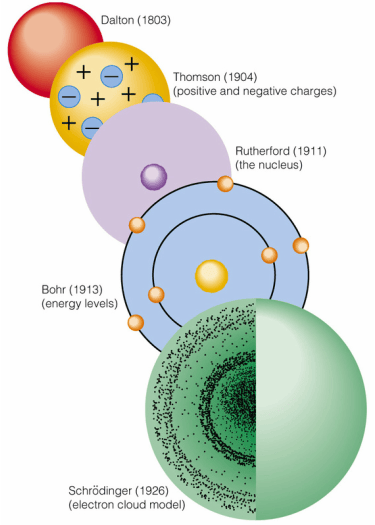
1. Dalton's Atomic Model (1803)
Description: John Dalton proposed that matter is composed of small, indivisible particles called atoms. He suggested that atoms of a given element are identical in mass and properties, and that chemical reactions involve the rearrangement of these atoms.
Limitations: Did not explain internal structure of atoms or the nature of chemical bonds.
2. Thomson's Plum Pudding Model (1904)
Description: J.J. Thomson discovered the electron and proposed that atoms are composed of negatively charged electrons embedded within a positively charged "soup," much like plums in a pudding.
Limitations: Could not explain the results of later experiments, such as Rutherford's gold foil experiment.
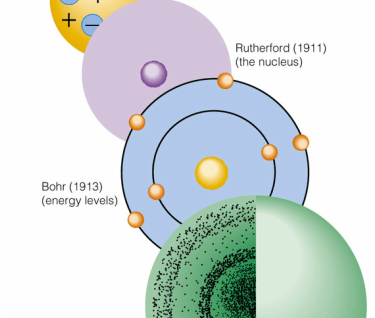
3. Rutherford's Nuclear Model (1911)
Description: Ernest Rutherford proposed that atoms consist of a small, dense, positively charged nucleus surrounded by electrons. He concluded this from his gold foil experiment, where alpha particles were deflected by the nucleus.
Limitations: Did not explain how electrons are arranged around the nucleus or why they do not spiral into the nucleus due to attraction to the positively charged nucleus.
4. Bohr's Model (1913)
Description: Niels Bohr suggested that electrons orbit the nucleus in fixed paths or energy levels without radiating energy. Electrons can jump between energy levels by absorbing or emitting photons.
Limitations: Explained the hydrogen atom spectrum well but failed to account for more complex atoms and the finer details of spectral lines.
5. Quantum Mechanical Model (1920s-Present)
Description: Based on the principles of quantum mechanics, this model treats electrons as wave-like entities that occupy orbitals rather than fixed orbits. Key contributors include Schrödinger, Heisenberg, and Dirac.
Schrödinger's Equation: Provides a mathematical framework to describe the probability distribution of electrons.
Heisenberg's Uncertainty Principle: States that one cannot simultaneously know the exact position and momentum of an electron.
Electron Cloud Model: Depicts regions of space (orbitals) where there is a high probability of finding an electron.
Features:
Orbitals: Regions where electrons are likely to be found (s, p, d, f orbitals).
Quantum Numbers: Define the properties of atomic orbitals and the electrons within them (n, l, m_l, and m_s).
Probability Distributions: Describes the likelihood of finding an electron in a particular region.
Summary of Key Concepts:
Dalton: Atoms are indivisible particles.
Thomson: Electrons embedded in a positively charged "soup."
Rutherford: Dense, positive nucleus with electrons orbiting.
Bohr: Electrons in fixed orbits with quantized energy levels.
Quantum Mechanical Model: Electrons in probabilistic orbitals, governed by wave functions and quantum mechanics principles.
Each successive model has built upon the previous ones, providing a more accurate and detailed understanding of atomic structure. The quantum mechanical model is the most current and widely accepted model, explaining the behavior of electrons in atoms and forming the basis for modern chemistry and physics.
