
क्या हार में क्या जीत में ! किंचित नहीं भयभीत मैं
संधर्ष पथ पर जो मिले यह भी सही वह भी सही।
वरदान नहीं मानूंगा, हार नहीं मानूंगा |
Bonding
CHEMISTRY
10/25/20222 min read
Bond Formation
There are several types of chemical bonding that occur between atoms to form molecules and compounds. These bonding types are determined by the sharing or transfer of electrons between atoms.
Bonding between atoms occurs primarily to achieve a more stable and lower energy state. The reasons why atoms bond with each other can be understood in terms of achieving stability through fulfilling the octet rule, minimizing energy, and gaining stability through attractive forces:
1. Stable Electron Configurations:
Atoms bond to achieve a more stable electron configuration, typically by gaining, losing, or sharing electrons.
Most atoms seek to achieve the electron configuration of noble gases, which have full outer electron shells (usually 8 electrons, except for helium which has 2).
2. Octet Rule:
The octet rule states that atoms tend to gain, lose, or share electrons in order to have a full set of 8 electrons in their outermost shell (valence shell).
By achieving a full outer shell, atoms become more stable and less reactive.
3. Lowering Potential Energy:
Bond formation typically leads to a more stable overall system with lower potential energy compared to isolated atoms.
When atoms bond, the potential energy of the system decreases because of the attractive forces that hold the atoms together.
The bonding on the basis of Sharing of electron:
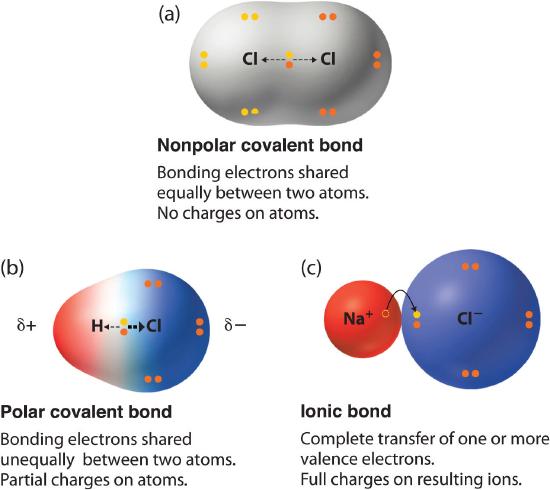
1. Ionic Bonding:
Ionic bonding occurs between atoms with significantly different electronegativities (large difference in their attraction for electrons).
In an ionic bond, one atom (typically a metal) loses electrons to become a positively charged ion (cation), while another atom (typically a non-metal) gains those electrons to become a negatively charged ion (anion).
These oppositely charged ions are held together by electrostatic forces, forming an ionic compound (e.g., NaCl).
2. Covalent Bonding:
Covalent bonding occurs between atoms with similar electronegativities (similar attraction for electrons).
In a covalent bond, atoms share pairs of electrons to achieve a stable electron configuration (usually the octet rule for main group elements).
Covalent bonds can be single, double, or triple, depending on the number of electron pairs shared between atoms.
Covalent bonds are typically found in molecules and non-metallic compounds (e.g., H2O, CO2).
3. Coordinate Bonding:
A coordinate bond, also known as a dative bond or coordinate covalent bond, is a type of covalent bond where both electrons shared between atoms come from one of the atoms involved in the bond.
coordinate bond is a special type of covalent bond where one atom (donor) provides both electrons to be shared in the bond, while the other atom (acceptor) does not contribute any electrons to the bond formation.
The atom that donates the electron pair is typically an atom with a lone pair of electrons that can be used to form the bond.
Coordinate bonds often form between Lewis bases (electron pair donors) and Lewis acids (electron pair acceptors).
The Lewis base (donor) has a lone pair of electrons that it donates to the Lewis acid (acceptor), which has an empty orbital to accept the electron pair.
Ammonia and Boron Trifluoride:
Ammonia (NH3) has a lone pair of electrons on nitrogen.
Boron trifluoride (BF3) has an empty p orbital and can accept a pair of electrons.
Ammonia donates a pair of electrons from its lone pair to BF3, forming a coordinate bond: NH3→BF3NH3 right arrow BF3NH3→BF3.
Hydronium Ion (H3O^+):
The hydronium ion is formed when a proton (H^+) coordinates with a water molecule (H2O).
Water donates a pair of electrons from its oxygen atom to form a coordinate bond with the proton: H2O→H+H2O right arrow H^+H2O→H+.
The Bonding on the basis of electronegativity differences
polar and non-polar bonding
Polar and nonpolar bonding refer to the types of covalent bonds based on the electronegativity difference between atoms sharing electrons.
Polar bonding
Polar bonding occurs when there is an unequal sharing of electrons between two atoms in a covalent bond.
This unequal sharing arises when one atom has a higher electronegativity (ability to attract electrons) than the other.
Characteristics:
In a polar covalent bond, the atom with higher electronegativity pulls the shared electrons closer to itself, acquiring a partial negative charge (δ⁻).
The atom with lower electronegativity acquires a partial positive charge (δ⁺) due to the electron imbalance.
As a result, polar molecules have a separation of electric charge (dipole moment) across the molecule.
Examples:
Water (H2O): Oxygen (O) is significantly more electronegative than hydrogen (H), leading to a polar covalent bond. The oxygen end of the molecule has a partial negative charge, while the hydrogen ends have partial positive charges.
Ammonia (NH3): Nitrogen (N) is more electronegative than hydrogen (H), resulting in a polar covalent bond and a dipole moment.
Nonpolar bonding
Nonpolar bonding occurs when there is an equal or nearly equal sharing of electrons between two atoms in a covalent bond.This occurs when the atoms involved in the bond have similar electronegativities.
Characteristics:
In a nonpolar covalent bond, electrons are shared equally or nearly equally between the atoms.
As a result, there is no significant separation of electric charge (no dipole moment) across the molecule.
Examples:
Diatomic molecules like hydrogen (H2), nitrogen (N2), and oxygen (O2) have nonpolar covalent bonds because the atoms in each molecule have the same electronegativity and share electrons equally.
Carbon dioxide (CO2) also exhibits nonpolar covalent bonds because the carbon-oxygen bonds are linear and symmetric, with equal sharing of electrons.
Summary:
Polar Bonding: Unequal sharing of electrons due to different electronegativities, resulting in partial charges and dipole moments (e.g., H2O, NH3).
Nonpolar Bonding: Equal or nearly equal sharing of electrons due to similar electronegativities, resulting in no dipole moment (e.g., H2, N2, O2, CO2).
Some other important bond
Metallic Bonding
Metallic bonding occurs within metals and alloys.
In metallic bonding, metal atoms release their valence electrons, creating a "sea" of delocalized electrons that are free to move throughout the structure.
These delocalized electrons are responsible for the high electrical and thermal conductivity, as well as the ductility and malleability, of metals.
Hydrogen Bonding:
Hydrogen bonding is a special type of intermolecular bonding that occurs between molecules containing hydrogen bonded to highly electronegative atoms such as nitrogen, oxygen, or fluorine.
It is a strong dipole-dipole attraction, where the hydrogen atom in one molecule is attracted to the electronegative atom in another molecule.
Hydrogen bonding plays a significant role in the properties of water (H2O) and in the structure of biological molecules such as DNA and proteins.
Van der Waals Forces:
Van der Waals forces are weak intermolecular forces that exist between all molecules, regardless of their polarity.
They include London dispersion forces, dipole-dipole interactions, and hydrogen bonding (as a special case).
Van der Waals forces increase with increasing molecular size and are responsible for the condensation and boiling points of substances.
These types of bonding are fundamental in understanding the properties and behavior of substances at the atomic and molecular levels. They govern the formation of chemical compounds, the interactions between molecules, and the physical properties of materials.
