
क्या हार में क्या जीत में ! किंचित नहीं भयभीत मैं
संधर्ष पथ पर जो मिले यह भी सही वह भी सही।
वरदान नहीं मानूंगा, हार नहीं मानूंगा |
Electrolysis
CHEMISTRY
10/25/20222 min read
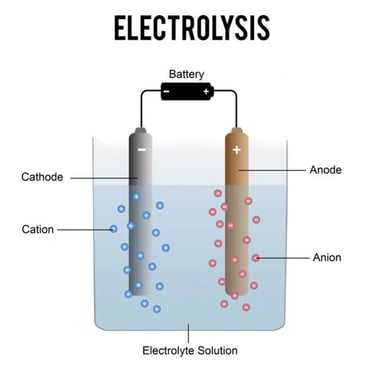
Electrolysis is a chemical process that uses electrical energy to drive a non-spontaneous chemical reaction. It is commonly used to decompose chemical compounds, particularly in the separation of elements from their naturally occurring sources, such as ores.
Basic Principles
Electrolyte: A substance containing free ions that make it electrically conductive. The electrolyte can be a molten ionic compound or an aqueous solution of an ionic compound.
Electrodes: Two solid conductors (typically made of metal or graphite) placed in the electrolyte. They are the anode (positive electrode) and the cathode (negative electrode).
Electric Current: When an external voltage is applied across the electrodes, it causes the ions in the electrolyte to move towards the electrodes of opposite charge, leading to chemical reactions at the surfaces of the electrodes.
Process Details
Anode Reaction (Oxidation): At the anode, anions (negatively charged ions) lose electrons (are oxidized).
Cathode Reaction (Reduction): At the cathode, cations (positively charged ions) gain electrons (are reduced).
Applications
Electroplating: Coating a metal object with a thin layer of another metal using electrolysis to prevent corrosion or for decorative purposes.
Electrorefining: Purifying metals (e.g., copper, aluminum) by removing impurities through electrolysis.
Electrolysis of Water: Splitting water into hydrogen and oxygen gases.
Production of Chemicals: For example, producing chlorine and sodium hydroxide from brine (saltwater solution).
Important Considerations
Overpotential: The extra voltage required to drive a reaction at a rate higher than the theoretical value due to kinetic barriers.
Electrode Materials: The choice of electrode material can affect the efficiency and selectivity of the electrolysis process.
Energy Consumption: Electrolysis is generally energy-intensive, so efficiency improvements and sustainable energy sources are important for practical applications.
