
क्या हार में क्या जीत में ! किंचित नहीं भयभीत मैं
संधर्ष पथ पर जो मिले यह भी सही वह भी सही।
वरदान नहीं मानूंगा, हार नहीं मानूंगा |
Fractional Distillation
Fractional distillation is a method used to separate mixtures of liquids with different boiling points
CHEMISTRY
10/25/20222 min read
Fractional distillation
Fractional distillation is a method used to separate mixtures of liquids with different boiling points. It is commonly employed in industries such as petroleum refining, chemical manufacturing, and the production of alcoholic beverages. This method enhances the separation efficiency by using a fractionating column, allowing for the separation of components that have relatively close boiling points. Here’s a detailed explanation of the fractional distillation process:
Principles of Fractional Distillation
Boiling Point Difference:
Fractional distillation separates components based on their boiling points. The liquid with the lowest boiling point vaporizes first.
Repeated Condensation and Vaporization:
The process involves repeated cycles of condensation and vaporization within a fractionating column, improving the purity of separated components..
Process
Heating:
The mixture is heated in the distillation flask. The component with the lowest boiling point vaporizes first.
Rising Vapor:
The vapor rises through the fractionating column. As it rises, it cools slightly and condenses on the packing material.
Condensation and Re-Vaporization:
The condensed liquid partially re-vaporizes as it absorbs heat from rising vapor. This cycle of condensation and re-vaporization continues up the column, enriching the vapor in the lower boiling point component.
Collection:
The vapor reaches the condenser, where it is cooled and condensed back into a liquid. This distillate is collected in the receiver flask.
Temperature Monitoring:
The temperature is carefully monitored. When the temperature starts to rise, it indicates that the next component with a higher boiling point is beginning to vaporize.
Sequential Collection:
By controlling the temperature and collecting distillates in separate fractions, each component of the mixture is separated.
Applications
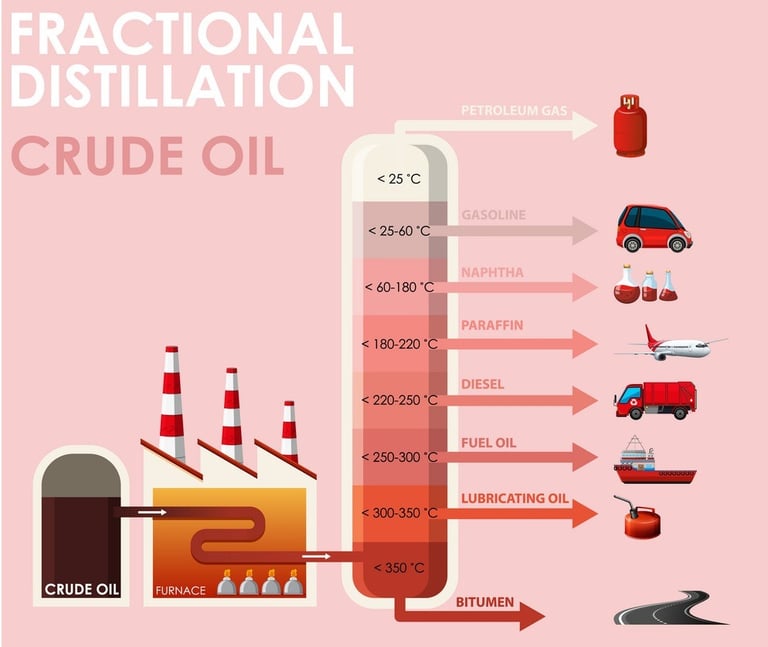
Petroleum Refining:
Separates crude oil into various fractions like gasoline, kerosene, diesel, and lubricating oils.
Chemical Industry:
Purifies chemicals and separates complex mixtures into individual components.
Alcoholic Beverage Production:
Used in the production of spirits to concentrate ethanol.
Air Separation:
Separates components of air (e.g., nitrogen, oxygen, argon) in cryogenic distillation.
Advantages
Efficiency:
Can separate components with close boiling points more effectively than simple distillation.
Purity:
Produces higher purity products due to multiple cycles of condensation and vaporization.
Scalability:
Suitable for both laboratory-scale and industrial-scale separations.
Limitations
Energy-Intensive:
Requires significant energy input, especially for components with high boiling points.
Complexity:
More complex and expensive setup compared to simple distillation.
Temperature Sensitivity:
Requires precise temperature control for effective separation.
Conclusion
Fractional distillation is a powerful technique for separating mixtures based on boiling point differences, particularly when the components have similar boiling points. Its ability to produce high-purity separations makes it indispensable in various industrial and laboratory applications.
