
क्या हार में क्या जीत में ! किंचित नहीं भयभीत मैं
संधर्ष पथ पर जो मिले यह भी सही वह भी सही।
वरदान नहीं मानूंगा, हार नहीं मानूंगा |
Fundamental particle of atom
CHEMISTRY
6/5/20241 min read
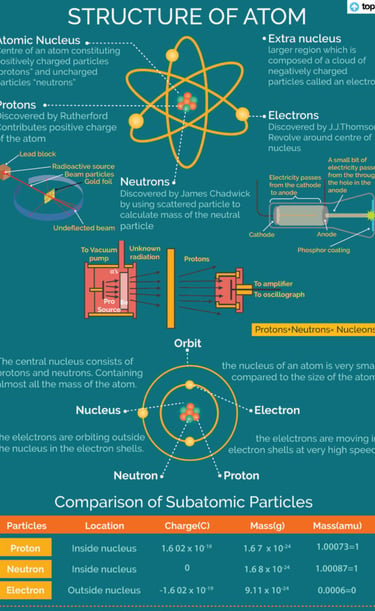
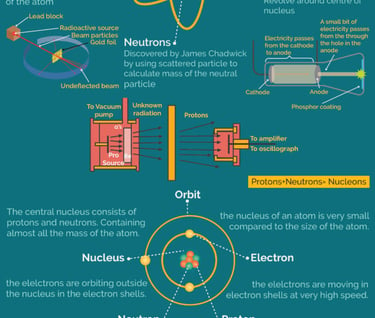
Fundamental particle of atom
Protons:
Protons are positively charged subatomic particles found in the nucleus of an atom.
Each proton has a relative charge of +1 and a mass of approximately 1 atomic mass unit (amu).
The number of protons determines the atomic number of an element, defining its chemical identity.
Neutrons:
Neutrons are neutral subatomic particles found in the nucleus of an atom alongside protons.
They have a mass similar to protons (approximately 1 amu) but carry no electrical charge.
Neutrons contribute to the atomic mass of an element and stabilize the nucleus by counteracting the repulsive force between positively charged protons.
Electrons:
Electrons are negatively charged subatomic particles that orbit the nucleus of an atom in electron shells or energy levels.
They have a much smaller mass compared to protons and neutrons (approximately 1/1836 amu).
The number and arrangement of electrons determine the chemical and physical properties of an element.
These three particles—protons, neutrons, and electrons—combine to form all known atoms. The protons and neutrons constitute the nucleus at the center of the atom, while electrons move around the nucleus in specific energy levels or orbitals. Understanding these fundamental particles is essential for comprehending atomic structure, chemical bonding, and the behavior of matter at the atomic level.
