
क्या हार में क्या जीत में ! किंचित नहीं भयभीत मैं
संधर्ष पथ पर जो मिले यह भी सही वह भी सही।
वरदान नहीं मानूंगा, हार नहीं मानूंगा |
Mole:
Used to express amounts of a chemical substance, a mole is the base unit of the amount of substance in the International System of Units (SI).
CHEMISTRY
6/25/20241 min read
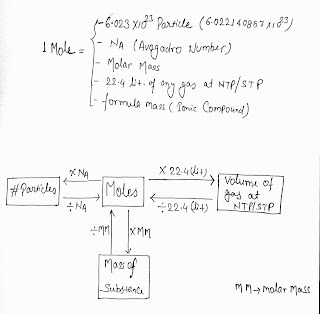
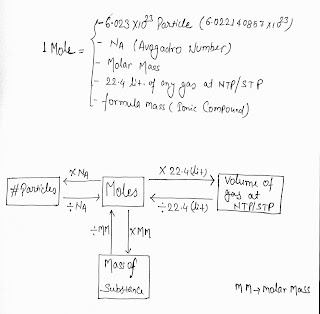
Mole is a number One mole is defined as the amount of a substance that contains as many entities as there are atoms in exactly 12 g of the C-12 isotope.
its exact value of 6.02214076×10 ²³ mol−1
In other word we can say that:
It is a fix quantity that may be in term of a number e.g. 6.023 X 10 ²³
or in terms of volume ( e.g. 22.4 liter ) at NTP and so on..
Question:
