
क्या हार में क्या जीत में ! किंचित नहीं भयभीत मैं
संधर्ष पथ पर जो मिले यह भी सही वह भी सही।
वरदान नहीं मानूंगा, हार नहीं मानूंगा |
The Aufbau principle
CHEMISTRY
6/25/20242 min read
Atomic Theory Contributions:
Determines the electron configuration of atoms, molecules or ions
Atoms are built up by continually adding electrons.
The term "Aufbau" is German for "building up," reflecting how electrons occupy orbitals starting from the lowest energy level to higher energy levels.
Key Points of the Aufbau Principle:
Electron Configuration:
Electrons fill orbitals starting with the lowest energy level and proceed to higher energy levels.
This "building up" process follows a specific order based on the increasing energy of orbitals.
Order of Filling:
The order in which orbitals are filled is determined by their relative energy levels.
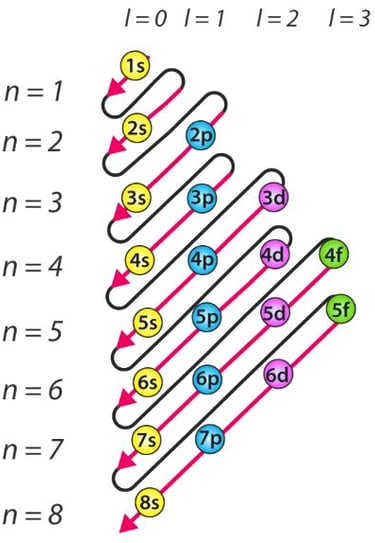
The sequence can be remembered using the following list or diagram, which shows the order of orbitals from lowest to highest energy:
Copy code
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p
Rules for Electron Filling:
Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, which must have opposite spins.
Hund's Rule: When electrons fill degenerate orbitals (orbitals of the same energy), they will singly occupy each orbital with parallel spins before pairing up.
Aufbau Principle: Electrons occupy the lowest energy orbital available.
Visualization of the Order of Filling:
A useful way to remember the order of filling is using a diagonal rule diagram. Start from the top of each column and move diagonally downwards to determine the sequence:
Copy code
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p
Example of Electron Configurations:
Hydrogen (H):
Atomic number = 1
Electron configuration: 1s¹
Carbon (C):
Atomic number = 6
Electron configuration: 1s² 2s² 2p²
Iron (Fe):
Atomic number = 26
Electron configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶
Neon (Ne):
Atomic number = 10
Electron configuration: 1s² 2s² 2p⁶
Exceptions to the Aufbau Principle:
While the Aufbau principle provides a general guideline, there are some exceptions, particularly among transition metals and heavier elements. These exceptions arise because the actual energy difference between some orbitals is very small, leading to configurations that maximize stability.
Chromium (Cr):
Expected: [Ar] 4s² 3d⁴
Actual: [Ar] 4s¹ 3d⁵
Copper (Cu):
Expected: [Ar] 4s² 3d⁹
Actual: [Ar] 4s¹ 3d¹⁰
These exceptions occur because half-filled and fully filled d subshells provide extra stability.
