
क्या हार में क्या जीत में ! किंचित नहीं भयभीत मैं
संधर्ष पथ पर जो मिले यह भी सही वह भी सही।
वरदान नहीं मानूंगा, हार नहीं मानूंगा |
Urea
CHEMISTRY
10/25/20222 min read
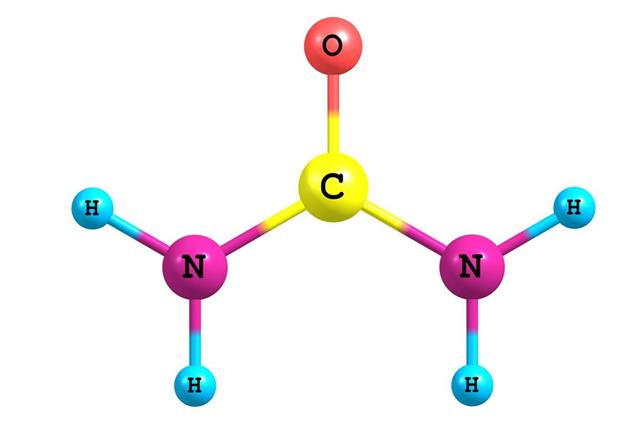
The first inorganic compound to be synthesized in a laboratory was ammonium cyanate (NH4OCN), which was converted to urea (CO(NH2)2) by Friedrich Wöhler in 1828.
Urea, also known as carbamide, is an organic compound. It plays a significant role in the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals.
Structure and Properties:
Physical Properties:
Appearance: White, crystalline solid
Melting Point: 133°C (271°F)
Solubility: Highly soluble in water
Synthesis:
Biological Synthesis (Urea Cycle):
Urea is produced in the liver through the urea cycle, a series of biochemical reactions that convert ammonia, which is toxic, into urea for excretion.
The cycle involves the conversion of ammonia and carbon dioxide into urea with the help of enzymes and ATP.
Industrial Synthesis:
Urea is synthesized industrially from ammonia and carbon dioxide under high pressure and temperature.
This process is known as the Bosch-Meiser urea process.
Uses of Urea:
Agriculture:
Urea is widely used as a nitrogen-release fertilizer due to its high nitrogen content (about 46%).
It is applied directly to the soil or used in various compound fertilizers.
Chemical Industry:
Urea is a raw material for the manufacture of many important chemicals, including plastics (such as urea-formaldehyde resins), adhesives, and pharmaceuticals.
Medical Applications:
Urea is used in dermatological products like creams and lotions to treat dry or rough skin conditions, as it helps to hydrate the skin.
Laboratory Uses:
Urea is used as a protein denaturant in biochemical research to study protein folding and structure.
Environmental Impact:
Nitrogen Cycle:
Urea plays a role in the nitrogen cycle. When used as fertilizer, it decomposes to ammonia and then to nitrate, which plants can absorb.
Excessive use can lead to environmental problems like water pollution due to runoff, causing eutrophication in water bodies.
Decomposition:
In the environment, urea breaks down into ammonia and carbon dioxide. The ammonia can be further converted into nitrate by soil bacteria through nitrification.
Health and Safety:
Urea is generally considered safe and non-toxic at normal exposure levels.
It can cause irritation to the eyes, skin, and respiratory tract if handled improperly.
Wöhler's Synthesis of Urea:
Background of urea :
Before Wöhler's experiment, it was believed that organic compounds could only be synthesized by living organisms through a "vital force." This theory, known as vitalism, posited a fundamental difference between organic and inorganic substances.
Summary:
Urea is a versatile compound with significant roles in both biology and industry. It is essential in the nitrogen cycle, used extensively in agriculture and the chemical industry, and has applications in medical and biochemical research. Its efficient production and utilization are crucial for sustainable agricultural practices and various industrial processes.
